Introduction: The treatment of chronic lymphocytic leukemia (CLL) has evolved over the past two decades, from finite duration chemoimmunotherapy (CIT) to an era of targeted agents such as Bruton Tyrosine Kinase (BTK) inhibitors, BCL2 inhibitors, and anti-CD20 monoclonal antibodies. Little is known about how initial management strategy (watch and wait [W&W] vs. treatment) at diagnosis impacts patient reported outcomes (PROs) in CLL survivors over time, particularly in light of therapeutic advances over the last decade.
Methods: Newly diagnosed CLL patients were prospectively enrolled within 9 months of diagnosis in the University of Iowa/Mayo Clinic SPORE Molecular Epidemiology Resource (MER1) study between 9/1/02 and 6/30/15, and within 6 months of diagnosis in the MER Phase 2 (MER2) study between 7/1/15 and 5/1/20. PROs were measured using the Functional Assessment of Cancer Therapy-General scale (FACT-G), which generates Physical (PWB, range 0-28), Social/Family (SFWB, 0-28), Emotional (EWB, 0-24), and Functional Well-Being (FWB, 0-28) scores and a Total Score (0-108), with higher scores indicating better WB. In both cohorts, FACT-G was measured at enrollment and at years 1, 2, and 3 after diagnosis, while the timing of later assessments varied slightly by study (MER1: 6 yrs and 9 yrs; MER2: 5 years). We employed a generalized linear mixed model to evaluate change from baseline between groups with groups defined by each participant's initial management strategy (W&W vs. upfront systemic treatment). Initial management strategy was determined by the treating physician. The study was approved by the Mayo Clinic IRB.
Results: A total of 722 patients were identified in MER1 and 256 patients in MER2. The median age at diagnosis was 63 years in MER1 and 66 years in MER2 (P<0.05); 66% were male in MER1 and 68% were male in MER2 (P>0.05). More patients initiated upfront systemic therapy in MER1 (n=380, 53%) than in MER2 (n=110, 43%). Other baseline clinical characteristics were similar between the cohorts ( Table 1). There was no statistical difference in the mean FACT-G total score at baseline between MER1 (85.0, SD 12.5) and MER2 (85.2, SD 12.7).
In the MER1 cohort, statistically significant lower baseline QOL scores were observed in patients initiating upfront treatment compared to W&W on the FACT-G total score (P=0.002) as well as the PWB (P=0.01), EWB (P=0.03), and FWB (P=0.006) subscales. We also observed that over time, EWB and FWB scores were consistently lower amongst those treated upfront compared to W&W. Further, EWB scores steadily improved over time regardless of initial management ( Figure 1), whereas the FWB and PWB subscales were largely stable over time. Mean SFWB scores dropped amongst patients who received upfront therapy at 3 years, which then steadily improved through year 9 but did not recover back to baseline. SFWB scores were stable among W&W patients.
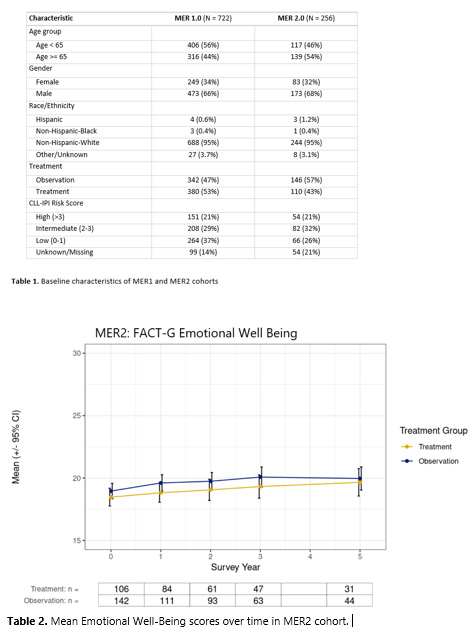
In the MER2 cohort, no statistically significant differences in any of the baseline QOL scores were noted between the W&W vs. initial treatment groups. Despite this, we observed consistently lower mean EWB scores among patients who initiated upfront treatment compared to W&W pts over time. No other statistically significant differences in mean QOL scores over time were observed between groups. Similar to MER1, we did observe a drop in mean SFWB scores amongst patients initiating upfront therapy at 3 years of follow-up that remained decreased at year 5.
Conclusion: This is the largest longitudinal study of CLL survivors that spanned over 18 years and two treatment eras. We demonstrated that in the novel treatment era, QOL metrics were similar between initial management strategies (W&W vs. treatment), suggesting that initial management had little to no impact on QOL at baseline or over time. In contrast, in the CIT era, we observed differences in QOL metrics at baseline between groups. In both treatment eras, patients with CLL who required upfront therapy demonstrated a decrease in SFWB from baseline at year 3 of follow-up, and the mean EWB scores were lowest at baseline regardless of initial management strategy (W&W vs. upfront treatment) or treatment era (CIT vs. novel agents), but steadily improved over time. These findings suggest opportunities for interventions to improve QOL in patients with CLL, and additional analyses are ongoing. Further follow-up in the era of novel agents is needed.
Disclosures
Koehler:AbbVie: Consultancy, Other: Advisory Board; Jannsen: Other: Advisory Board; Astra Zeneca: Other: Advisory Board. Parikh:Juno Therapeutics: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Accerta Pharmaceuticals: Research Funding; Bristol Myers Squibb-Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Beigene: Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding; Boehringer Ingelheim Pharmaceuticals Incc: Membership on an entity's Board of Directors or advisory committees; Dren Bio: Membership on an entity's Board of Directors or advisory committees; Dava Oncology: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie Inc: Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Agios Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Sunesis: Research Funding; Vincerx: Research Funding. Shanafelt:Pharmacyclics: Research Funding; AbbVie: Research Funding; Genentech: Research Funding. Tsang:Poseida Therapeutics: Current holder of stock options in a privately-held company. Bommier:INSERM: Current Employment; ITMO/AvieSan: Research Funding; LYSA/ELI: Research Funding; Philippe Foundation: Other: Mobility funding; Institut Servier: Research Funding. Cerhan:NanoString: Research Funding; Protagonist: Other: Safety Monitoring Committee; Genmab: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal